

The maximum electrons holding capacity in M orbit is 2n 2 = 2 × 3 2 = 18. The maximum electron holding capacity in L orbit is 2n 2 = 2 × 2 2 = 8. The maximum electron holding capacity in K orbit is 2n 2 = 2 × 1 2 = 2. The electron holding capacity of each orbit is 2n 2. K is the name of the first orbit, L is the second, M is the third, and N is the name of the fourth orbit. These circular paths are called orbit(shell). The electrons of the atom revolve around the nucleus in a certain circular path. The complete idea of the orbit is given there. Scientist Niels Bohr was the first to give an idea of the atom’s orbit. Sodium atom electron configuration through orbit Determination of the valency and valence electrons.Determining the block of sodium by electron configuration.Determination of group and period through electron configuration.
#Sodiums atomic radius how to
How to write the orbital diagram for sodium?.Electron configuration of sodium through orbital.
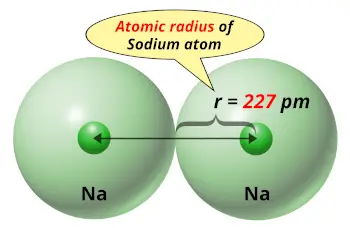

The electron configuration of a sodium atom can be done in two ways. The arrangement of electrons in different orbits and orbitals of an atom in a certain order is called electron configuration. These electrons are arranged according to specific rules of different orbits. The total number of electrons in sodium is eleven. Sodium is a classified alkali metal element. Sodium is the 11th element in the periodic table and its symbol is ‘Na’.


 0 kommentar(er)
0 kommentar(er)
